
Non sterile isolation gown
- Packung mit 100 Stück Packung mit 100 Stück

Nous contacter : 01 48 01 32 89
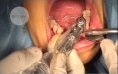
HYGITECH Academy invites you to watch this clinical case on dental implant placement after bone...
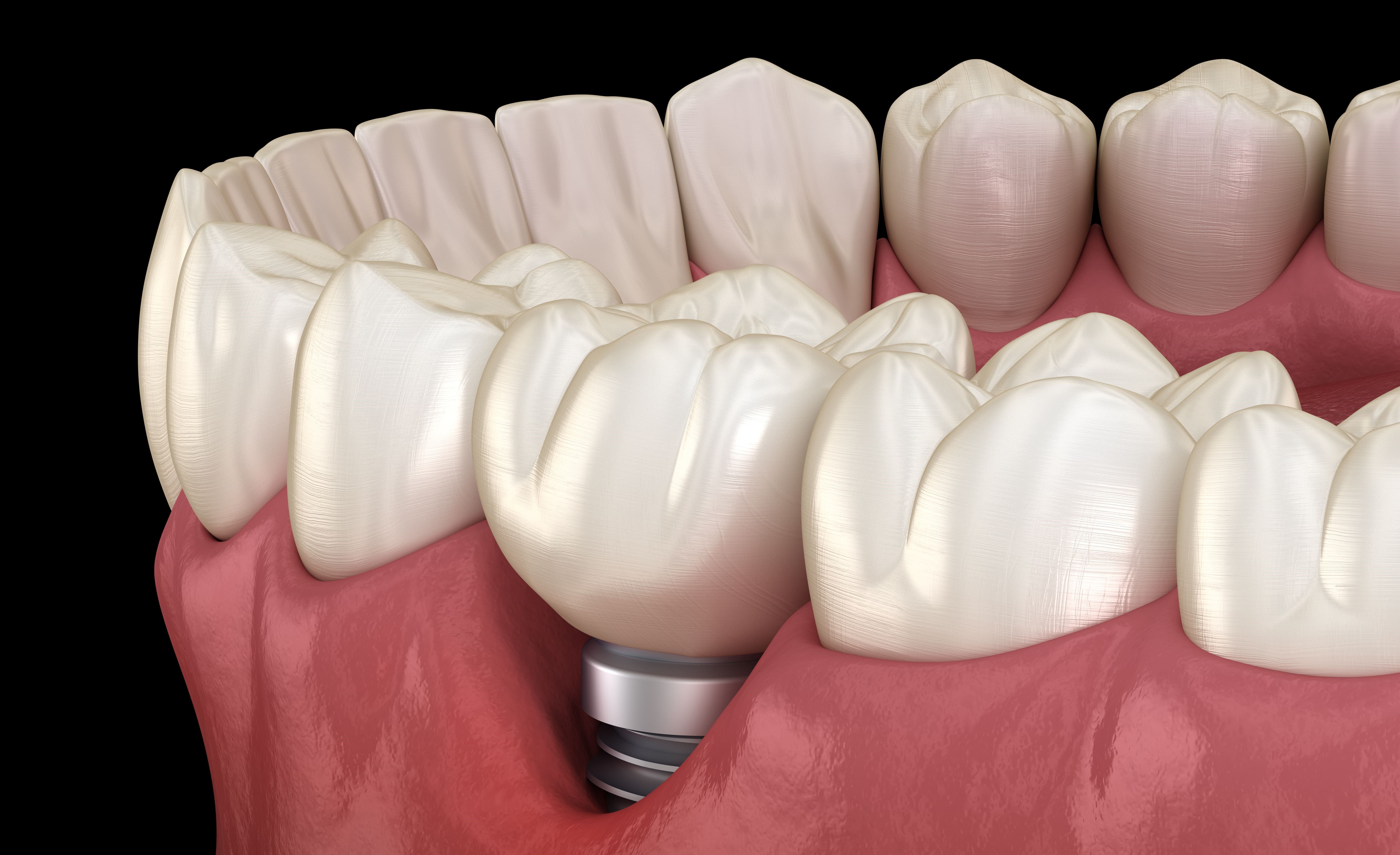
Further to the anatomical-histological review of periodontal and peri-implant tissues, this...

Implant unit motors are necessary equipment for placing dental implants. They are composed of a...
Introduction
Dental implantology, as an invasive surgical procedure, is strictly governed by national and European regulations. These requirements apply to practitioners, healthcare facilities, as well as medical device manufacturers and distributors. Compliance with these obligations is crucial to ensure patient safety, maintain traceability of procedures, and protect against legal liability.
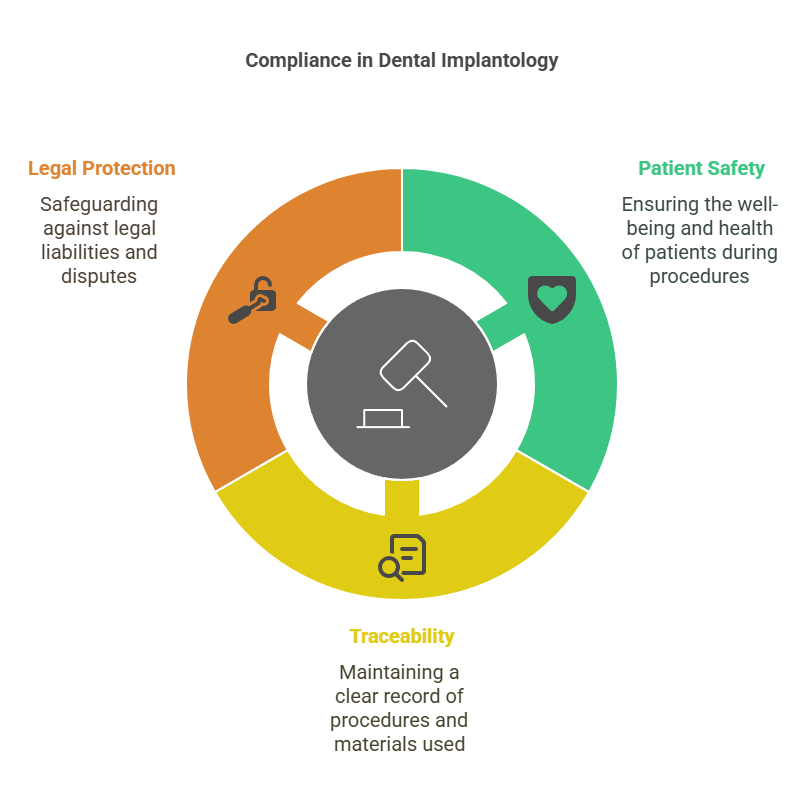
According to the French Public Health Code (Article R.4127-204), every dental surgeon must ensure they possess the necessary skills to perform implant procedures. Continuing education in implantology is therefore a deontological duty and, implicitly, a legal requirement under the duty of care.
Important: Performing implant surgery without appropriate training can result in professional liability in case of complications.
Before any procedure, the practitioner must provide the patient with clear, honest, and appropriate information regarding risks, benefits, therapeutic alternatives, and postoperative care (Article L.1111-2 of the Public Health Code). Ideally, this information should be documented in writing (signed informed consent).
The surgical environment for implantology must comply with strict aseptic standards, based on the HAS (French Health Authority) recommendation dated October 24, 2019, concerning "Conditions for Performing Oral Implantology Procedures":
A dedicated area for surgical acts,
Sterile surgical attire (gloves, mask, cap, gown),
A rigorous disinfection and sterilization protocol.
With the entry into force of Regulation (EU) 2017/745 on Medical Devices (MDR), safety, traceability, and technical documentation requirements for dental implants have been significantly reinforced.
CE Certification and Traceability
Every implant or component used (abutments, screws, drills, etc.) must:
Bear the CE mark,
Be accompanied by a traceability record to be kept in the patient’s medical file,
Be registered in the Implantable Devices Register (UDI system), ensuring traceability both upstream (supplier) and downstream (patient).
Documentation and Labeling
Manufacturers and distributors, such as HYGITECH, must provide:
A compliant instructions manual,
Multilingual labeling,
A vigilance system to report any incidents or adverse events.
Implantable devices are subject to a traceability requirement for at least 10 years (Article R.5212-36 of the Public Health Code). The practitioner must document:
The batch references of the implant used,
The date and circumstances of placement,
Any postoperative outcomes and potential complications.
A structured and updated medical file at each treatment stage is essential to meet this requirement.
Conclusion: Compliance is Essential at Every Level
Compliance with legal obligations in implantology engages the responsibility of practitioners, manufacturers, and distributors. These requirements are not only regulatory: they are central to ensuring safe, ethical practice and maintaining the quality of care.
Sterile surgical kit → View product
Sterile drapes → View product

 How to choose an implant unit?
How to choose an implant unit?
 How to choose an autoclave?
How to choose an autoclave?
 List of surgical goods and equipment needed
List of surgical goods and equipment needed
