
Protection sleeve 90 cm with adhesive for tubing
- Packung mit 250 Packung mit 250

Nous contacter : 01 48 01 32 89
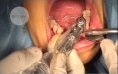
HYGITECH Academy invites you to watch this clinical case on dental implant placement after bone...

In dental practices, mastering the aseptic chain is crucial for preventing cross-infections. Among the critical steps, surface decontamination plays a central role. Work surfaces, equipment, and furniture can act as indirect vectors for pathogen transmission, including viruses, bacteria, and spores. A rigorous cleaning strategy is therefore essential, especially in high-risk contamination areas.
Procedures performed in dental surgery, particularly implantology, generate aerosols and splashes that can contaminate nearby surfaces (dental chair, countertops, care units, operating lights, etc.). According to the CDC (Centers for Disease Control and Prevention), some bacteria, such as Staphylococcus aureus or Clostridium difficile, can survive on inanimate surfaces for several days, or even weeks, if not properly disinfected.
The HAS (French Health Authority) emphasizes that environmental contamination can contribute to the transmission of multidrug-resistant organisms, especially in the presence of soiled equipment or structural defects.
Thus, adopting a strict, standardized, and traceable protocol is essential.
Two types of surfaces require disinfection:
Critical and semi-critical surfaces: those in direct or indirect contact with the patient or instruments (e.g., trays, syringes, handpieces). They must be disinfected between each patient.
Non-critical surfaces: those rarely exposed to biological fluids (e.g., floors, door handles, light switches). They should be disinfected daily, or more frequently depending on activity.
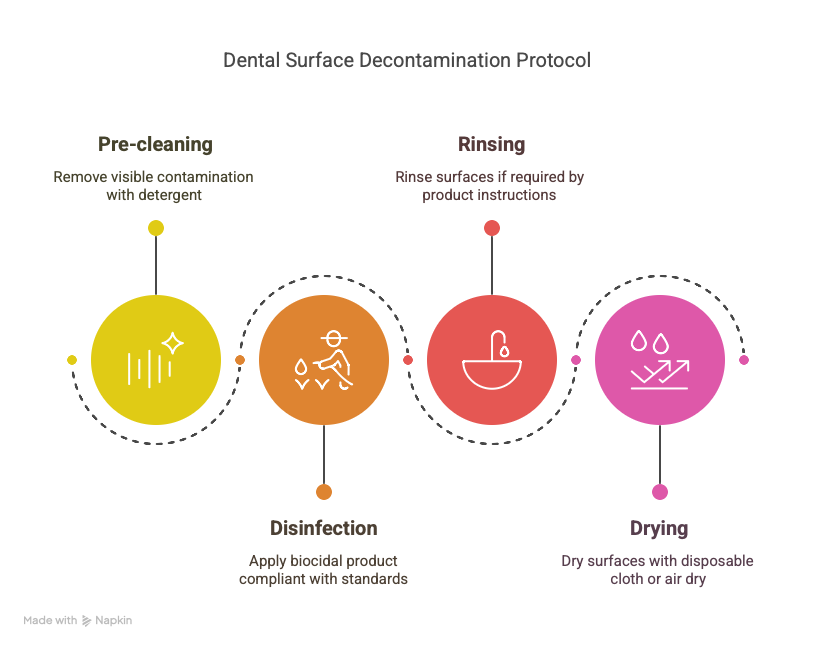
The decontamination protocol must follow these steps:
Pre-cleaning: remove visible contamination using a non-disinfectant detergent or a detergent-disinfectant product, applied with disposable cloths or wipes.
Disinfection: use a biocidal product compliant with EN 14476 (virucidal activity), EN 13727 (bactericidal), and EN 13624 (fungicidal) standards. Strictly respect the contact time indicated.
Rinsing if necessary: some products require rinsing after application, especially on sensitive surfaces (e.g., instruments, screens).
Drying: dry with a disposable cloth or allow to air dry according to the manufacturer's instructions.
It is crucial to use products compatible with the materials to avoid damage. Single-use application devices further limit cross-contamination risks.
Wearing PPE: gloves, goggles, and masks must be worn during cleaning.
Traceability: maintaining a logbook or tracking sheet helps document cleaning/disinfection activities.
Ongoing training: staff should receive regular training aligned with official recommendations.
Use of protective films: for surfaces that are difficult to clean (e.g., keyboards, touchscreens), the use of single-use barrier films is recommended.
Surface decontamination should not be viewed as a constraint but as a fundamental lever for ensuring patient safety.
Rigorous monitoring, clear organization, and strict adherence to validated protocols effectively reduce the risk of cross-contamination.


 Sterilization room layout
Sterilization room layout
